Ion exchange is a water treatment commonly used for water softening or deminerialization and to remove other substances from water.
Ion exchange method for water treatment ppt.
This soft mineral is contained on the softener resin beads and does not build up on surfaces as scale.
10 ion exchange process 1.
Treatment but chemical mechanism is ion exchange rather than adsorption.
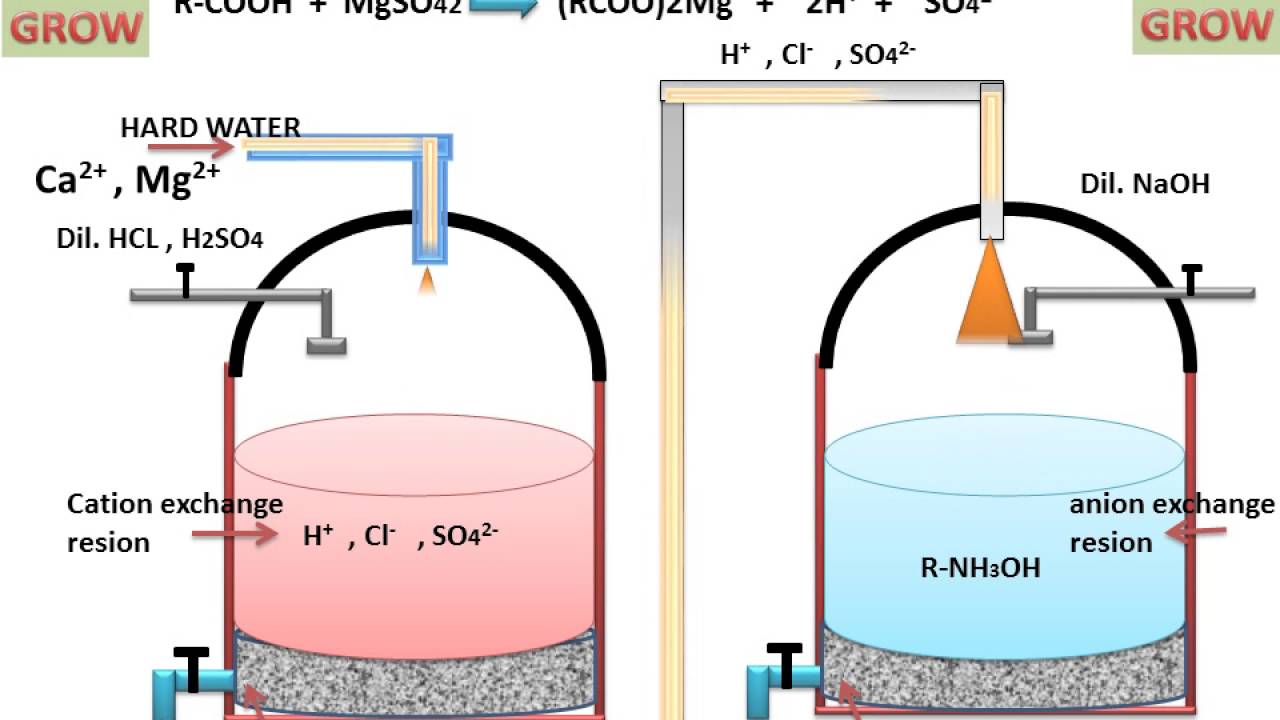
The positive or negative ions fixed on these radicals are replaced by ions of the same sign in solution in the liquid in contact with them.
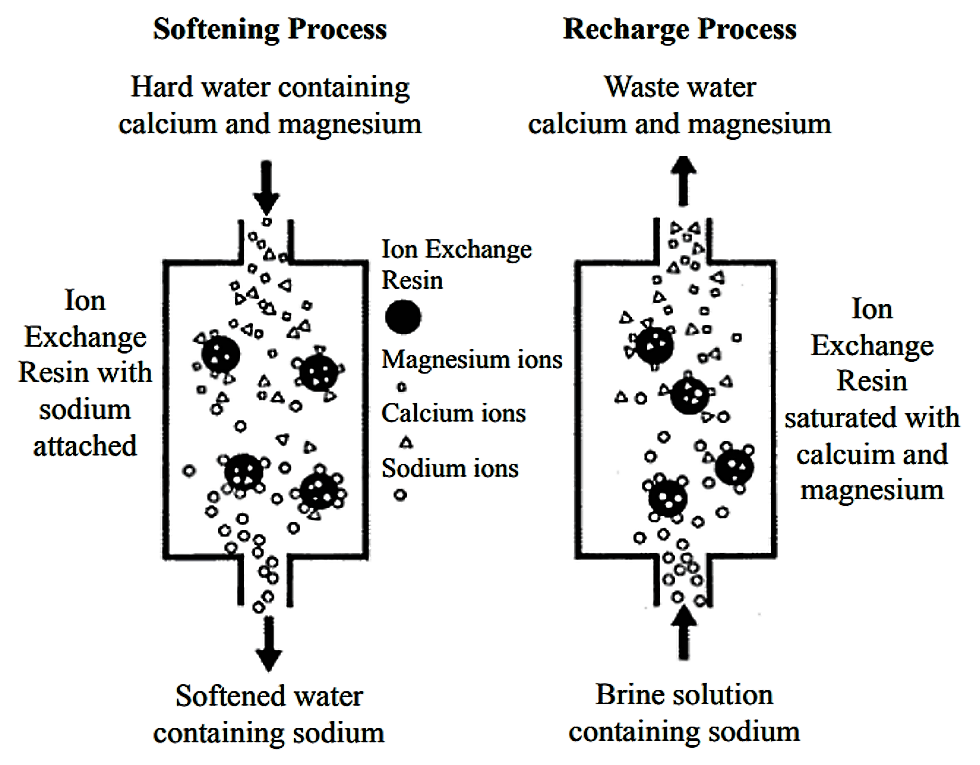
Sodium ions are supplied from dissolved sodium chloride salt also called brine.
Pellicular type with ion exchange film.
In the ion exchange process a granular substance usually a resin that is coated with sodium or potassium ions comes into contact with water containing calcium and two positively charged sodium or potassium ions are exchanged released into the water for every calcium or magnesium ion that is held by the resin.
0 01 0 1 meq g.
Water softeners usually use sodium na as the exchange ion.
Dvorak extension environmental engineering specialist the presence of calcium ca and or magnesium.
The particles have a size of 30 40µ with 1 2µ film thickness.
Ions electrically charged atoms or molecules cations and anions can have one or more charges mostly 1 to 3 and can be made of one or.
Water softening ion exchange sharon o.
Skipton extension water quality educator bruce i.
In the ion exchange process sodium ions are used to coat an exchange medium in.
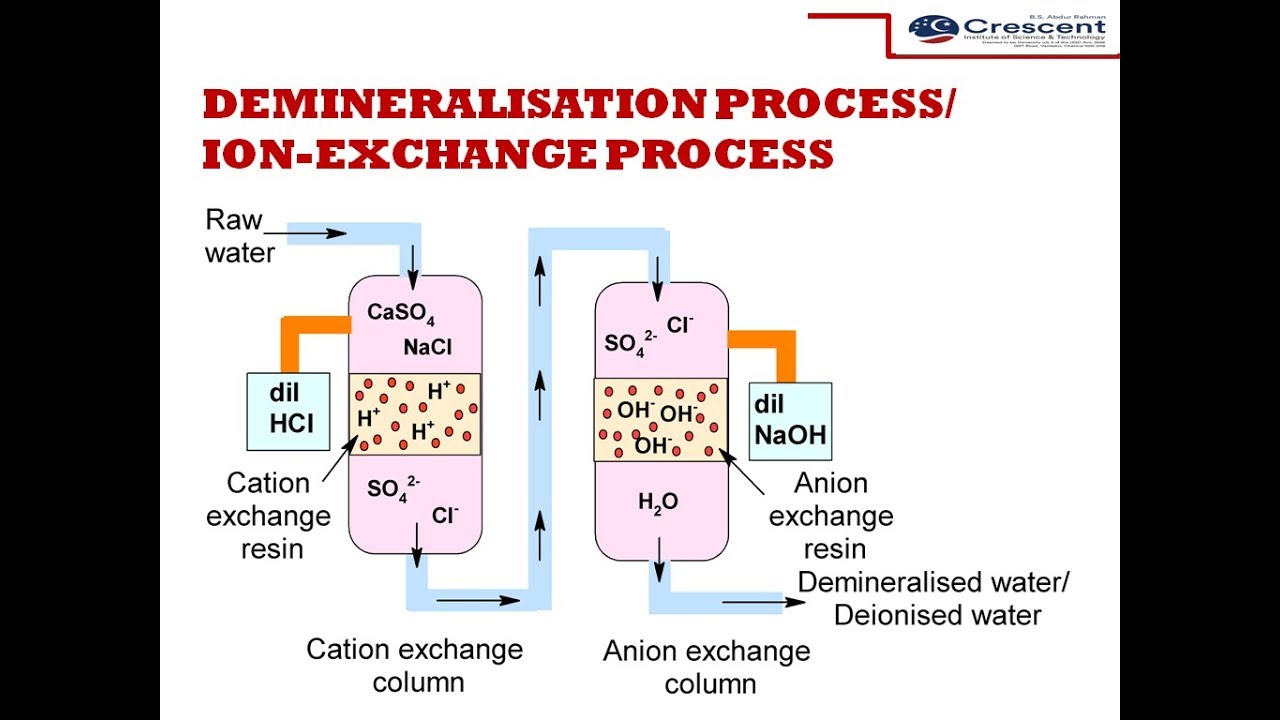
An ion exchanger is an insoluble substance containing loosely held ions which can be exchanged with other ions in solution which come in material.
The two most common ion exchange methods are.
An ion exchange water softener exchanges the hardness minerals calcium and magnesium dissolved in water for sodium.
Ions in the water are exchanged for other ions fixed to the beads.
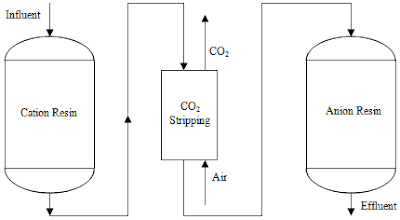
Ion exchange is a water treatment process commonly used for water softening or demineralization but it also is used to remove other substances from the water in processes such as dealkalization deionization and disinfection.
Ion exchange process 2.
These have very low exchange capacity ion exchange efficiency.